From the mid-1960s, controlled potential electrolysis type sensors have attracted much attention as a means for sensing and measuring air pollutants such as sulphur dioxide and nitrogen oxide right at the source where they are generated and they are increasingly being used in such contents. In the latter half of the 1970s, they came to be more widely accepted as carbon monoxide or hydrogen sulphide measuring instruments alongside other combustion type or galvanic sensors; not only for flue gas monitoring or occupational hygiene and safety applications.
Entering the 1980s, amid technical progress and development of new industries, the trend was towards enhanced sensitivity of existing sensors which was proactively pursued while the application range was also expanded greatly as sensors were developed for unusual gases, acidic gas, halogen, etc.
- An extremely wide range of gases can be sensed and measured
- High susceptibility to interference by similar gases, making individual measurement of gases of the same family quite difficult at times
- Selectivity can be enhanced by changing the electric potential, the electrode material, and the electrolyte solution
Function principle
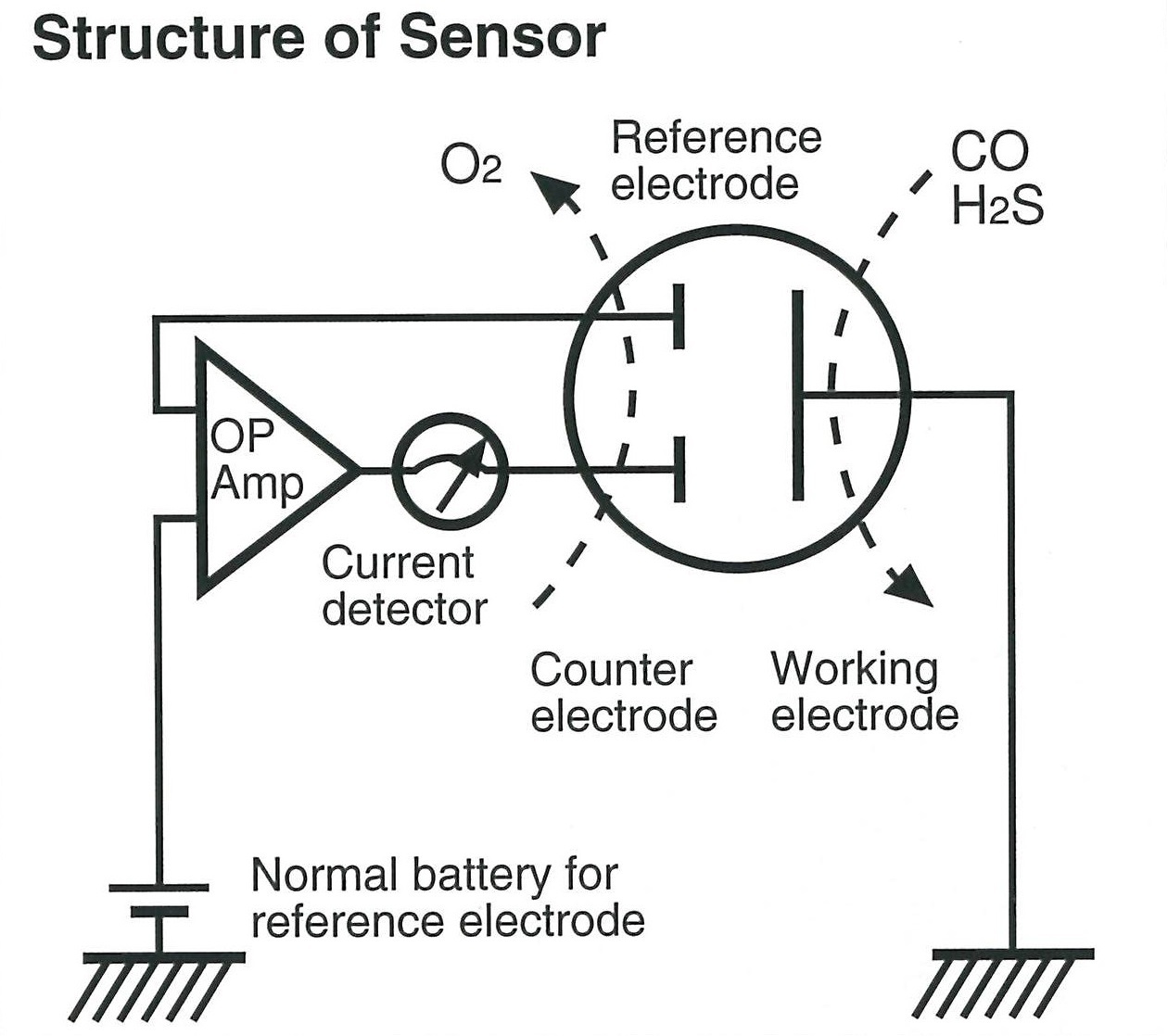
Essentially, it consists of a sealed plastic container that comprises a gas permeable film, a work electrode, a reference electrode, an opposite electrode, and an electrolyte solution. The function principle essentially is as follows: the potential of the work electrode vis-a-vis the reference electrode is regulated as electrolysis takes place, measuring the electrolytic current that flows at that time, in order to determine the concentration of the target gas. To take the example of CO (carbon monoxide), the oxidation reaction shown in equation (1) takes place in the working electrode and a redox process (2) occurs in the opposite electrode with equation (3) expressing the entire chemical reaction. At this time, the current that flows to the working electrode and the opposite electrode respectively is proportional to the concentration of the carbon monoxide present
(1)
Carbon monoxide : CO + H2O → CO2 + 2H+ + 2e-
Hydrogen sulphide : H2S + 4H2O → H2SO4 + 8H+ + 8e-
(2)
Carbon monoxide : 1/202 + 2H+ + 2e- → H2O
Hydrogen sulphid : O2 + 4H+ + 4e- → 2H2O
(3)
Carbon monoxide : CO + 1/2O2 → CO2
Hydrogen sulphide : H2S + 2O2→ H2SO4
*The products may not be sold depending on countries and regions.
Please use the relevant link below to make an inquiry.
Product search
-
Detector tube Search
-
Search other products
-
Product search