Q.1
Why does the gas detector tube change colour?
A.1
The detector tube contains a chemical known as a reagent. For example, in the carbon dioxide detector tube, it is the white portion of the filling, and in the oxygen detector tube it is black. When the target gas and the reagent come into contact, a chemical reaction takes place that causes the colour to change. In other words, as soon as the target gas (to be measured) passes through the detector tube, the colour changes that show the presence of the target gas and enables its concentration to be known.
Or, to explain it in more detail:
The actual reason for the colour change is that the reagent substance undergoes a chemical reaction upon contact with the gas so that it is transformed into a chemically different substance.
For instance, when the white reagent in the carbon dioxide detector tube comes into contact with carbon dioxide, it is transformed into a differently constituted purple substance. Or, when the black reagent in the oxygen detector tube comes into contact with oxygen, it is transformed into a differently constituted white substance.
(There are also some detecting reagents that use a slightly different process to change colour than that described above).
Q.2
Why is the length of the colour change in the gas detector tube different, depending on the concentration of the target gas?
A.2
First of all, the detector tube contains a detecting regent (as already explained in the answer to the previous question: "Why does the gas detector tube change colour?").
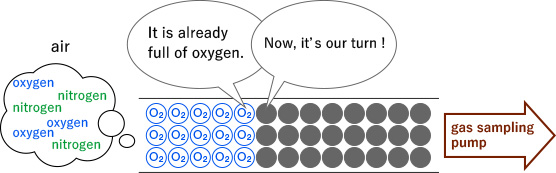
Now, let us use the oxygen detector tube as an example. It contains tightly packed grains of the reagent powder as shown in the drawing below.
During measuring, air is drawn (sucked) into the detector tube using a sampling pump. The oxygen molecules in the air adhere to the grains of the reagent causing them to change from black to white.
One row at a time, the oxygen molecules advance through the reagent grains adhering to them as it penetrates deeper and deeper into the tube. This process continues, layer for layer, until there is no more oxygen and the colour change stops.
Essentially, differing gas concentrations mean different amounts of oxygen in the air that is drawn (sucked) through the detector tube with the sampling pump. When there is lots of oxygen (i.e. a high concentration), the oxygen molecules advance very far through the reagent (leaving a long strip of white), whereas when there is little (i.e. low concentration) it is quickly used up as it passes through and reacts with the reagent; so, the strip of changed colour (white) tends to be shorter.
Hence, the length of the discoloured strip in the detector tube, depends on the concentration of target gas in the air to be tested/measured.
Q.3
Why does the oxygen detector tube become hot during measurement?
A.3
If you have ever used a disposable heat pack to keep warm during the winter cold, you have seen how it suddenly becomes warm after opening the bag and shaking it. This is because of the iron-based chemical substance used: oxygen molecules adhere to its individual grains and generate heat in the process.
A substance known as titanium is used in the oxygen detector tube. Besides changing colour when the oxygen molecules adhere to the titanium grains, heat is also given off (similar to the disposable heat pack).
Q.4
What is the "ISO"?
A.4
Many people mistakenly believe ISO stands for "International Standards Organization" or something like that. However, ISO is NOT an acronym (abbreviation); it comes from the Greek word isos, which means ÒequalÓ. Its English name is International Organization for Standardization, while in French it is called Organisation Internationale de Normalisation; so using an acronym would require different ones in different languages (for example: IOS in English, or OIN in French). Since, this would be contrary to the idea of standardization, the founders of the organization chose ISO as the sole official short form of its name. The ISO was established in 1947 with its headquarters in Geneva (Switzerland). Today, over 150 countries are cooperating and participating in the organization and its activities to harmonize standards and rules around the world.
As long as they are strictly adhered to, common standards enable us to buy a product in one country and use it all over the world. For instance, if we take our camera on a trip overseas we can buy film anywhere because it is all the same size and type wherever we may go. Without such common standards, the film might not work or even fit in our camera. As long as they are strictly adhered to, common standards enable us to buy a product in one country and use it all over the world. For instance, if we take our camera on a trip overseas we can buy film anywhere because it is all the same size and type wherever we may go. Without such common standards, the film might not work or even fit in our camera.
Q.5
What is "ISO9001"?
A.5
ISO9001 is an international standard for quality control with which manufacturers ensure customers that their products are properly and consistently made to a good quality. Basically, ISO 9001 sets down the system that enables this quality and reliability to be maintained. (By "system" we mean the entire processes and regulations for the manufacture and selling of products.
For a company to receive ISO9001 certification (i.e. approval), it must prove that its production and related processes meet the necessary requirements. Special nationally and internationally recognized organizations in various countries inspect companies and their facilities periodically to check that the ISO9001 requirements are being met. So, the ISO9001 certification shows that a company has the necessary system in place and that its products are reliable.
GASTEC Corporation received ISO9001 certification in April 1998.
Q.6
When was the detector tube invented?
A.6
1919
The first gas detector tube (for detecting carbon monoxide) was invented by Professors Hoover and Lamb of Harvard University in the United States. It was used to detect carbon monoxide.
1946
Professor Kitagawa developed Japan's first concentration-chart based detector tube for hydrogen sulphide. A calibrated scale printed on a ruler-like chart was held up against the discoloured section of the tube to obtain the concentration reading.
1970
GASTEC developed Japan's first direct reading detector tube and an easy-to-use gas sampling pump. The GASTEC detector tube was the first one in Japan to have a calibrated scale (for reading the gas concentration) directly printed on the tube.
1992
Across Japan, many primary schools began to use GASTEC gas detection kits (including detector tubes and sampling pump) for experiments in 6th grade science classes.
Q.7
Where are detector tubes in use?
A.7
In education
in the classroom, lab, and for school safety
At schools they are used as teaching aids, in lab experiments, or to monitor the quality of the air. Perhaps there are even some GASTEC gas detector tubes at your school.

In the home
gas leaks, burner trouble, building material fumes.
In many homes gas is used for cooking, heating, etc. and leaks or incomplete combustion (burning) of gas can pose a hazard. Then, there are some building materials from which toxic fumes (for example, formaldehyde) are slowly given off. Detector tubes can be very useful to detect them.
In heavy industry
including steelmaking, shipbuilding, automotive, pulp/paper production.
In production
of food, consumer electronics and other products.
At chemical and energy related facilities
for example: chemical industry, oil refineries, gas, and electric power utilities, etc.
Is there a big factory in (or near) your neighborhood? Perhaps, there is an iron foundry, a shipbuilding yard, an automobile plant, a paper mill or some other type of factory? Many production or energy generating processes require special gas and/or waste gas is given off which may be harmful. By controlling the amounts (i.e. concentrations) of such gases, better products can be produced with less harm to the environment or to people working and living nearby. Detector tubes come in very handy for the monitoring and measuring that is necessary.
At construction sites
for waterworks, sewerage, gas, and other facilities/buildings.
Sometimes there is hazardous gas at a construction site; for example, during excavation work there may be toxic fumes at the bottom of the hole. Detector tubes help to check this and warn us which helps to prevent accidents.
Research and development facilities
including research/experimental labs, etc.
Sometimes there is hazardous gas at a construction site; for example, during excavation work there may be toxic fumes at the bottom of the hole. Detector tubes help to check this and warn us which helps to prevent accidents.
In transportation
trains/subways, ferries, airplanes, buses, etc.
At medical facilities
hospitals, clinics, infirmaries, etc.
In offices
work stations, conference rooms, reception/lobby areas, studios, etc.
During sports/leisure activities
swimming pools, athletic gyms, aerobics studios, etc.
In public areas and facilities
theatres (movie/stage), concert halls, department stores, hotels,
It is important to be sure that air quality is good wherever there are lots of people. To do this, we must first know if and how dirty the air gets there. So, careful inspection and measurement with precise means such as GASTEC detector tubes is important.
The examples we have listed on this page are only some of the many uses for our gas detector tubes that help so many people live safe and healthy lives.
-
Experiments with Detector Tubes
-
Let's think together

